1008
Views & Citations8
Likes & Shares
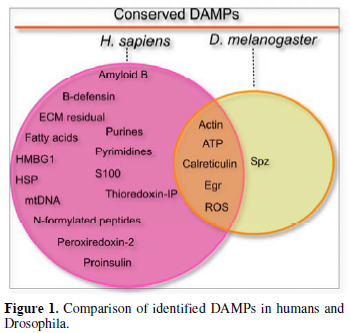
Insects, including the fruit fly, Drosophila melanogaster are used to study a wide array of processes, many of which are known or are expected to be regulated by damage-associated molecular patterns (DAMPs). These include regenerative processes after wounding, replacement of cells by cell competition, induction of immunity and inflammation, responses against tumorous cells and neurodegeneration. Most, if not all of these processes have beneficial outcomes on organismal health but may also lead to pathologies, which often resemble those observed in humans. Drosophila offers unique opportunities to analyze and manipulate genes and pathways related to these immune consequences with high temporal and local resolution. Ultimately, such detailed analyses in the Drosophila model will aid in our understanding of the roles DAMPs play at the bifurcation between physiological and pathological outcomes in other animal species, including humans.
Keywords: Coagulation, Danger signals, DAMPs, Hemocytes, Inflammation, Innate immunity, Insect immunity, Regeneration, Tumors, Wound healing
INTRODUCTION
DROSOPHILA IMMUNITY - A QUICK GUIDE
WOUND SEALING AND HEALING: LIVE IMAGING AND BEYOND
In an infection context, hemolymph clots have been demonstrated to prevent entry of parasites that target epithelial surfaces using mechanical tools such as entomopathogenic nematodes (EPNs), which use their mouth part to gain entry to the hemocoel [4]. EPN infection leads to a massive induction of immune-related genes although some immune genes appear to depend on the clot or clot components for their induction rather than on microbial or parasite-specific elicitors [17] and thus are more akin to microbe-independent responses like those observed during sterile inflammation. In microarray data of Drosophila infected with the EPN Heterorhabditis bacteriophora, it was found that several hundred genes are specifically induced in EPN infection in comparison to other types of infections, like parasitic wasp infection, and that there are several candidate damage-induced molecules such as thioester containing protein-1, Eiger, Spätzle-processing enzyme and potentially others that are yet to be characterized [17,18]. Interestingly, the Toll reporter Drosomycin is highest on that list providing evidence for alternative ways of inducing AMPs independent of the Toll pathway. Similarly, Hauling et al. [19], through RNA sequencing of the fat body gained further insights into the endogenous response against danger signals produced from tumor-expressing salivary glands [19]. Beyond the current molecular scope, we have successfully been implementing the use of sequencing data as a map for finding new danger signals and DAMPs.
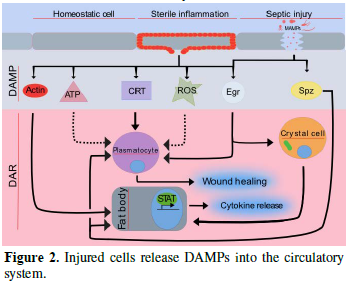
Released DAMPs enable broad trans-tissue communication. In external wounding situations, local DAMPs at wound sites establish local communication between the wound and the immune system to ensure that a proper wound healing response is taking place. In injured organs, actin can be exposed on the surface of the wound site [20]. Similar findings have revealed that actin is also found in the blood, suggesting that it may be released from injured tissue [21]. However, the mechanism of DAMP-actin-induced inflammation is not fully understood. Another role actin may have is to serve as a signal molecule [22]. For instance, Ahrens et al. [23] found that actin is a danger signal that is conserved from yeast to humans. F-actin acts as a ligand and is recognized by a DAMP receptor for dead cells called DNGR-1 (also known as CLEC9A) in both vertebrates and Saccharomyces cerevisiae [23]. In mosquitoes, actin has been found to promote phagocytosis of bacteria in cooperation with the small MD2-like protein and to act as a Plasmodium falciparum antagonist [22]. Recently, Srinivasan et al. [24] identified actin as a conserved DAMP in the fruit fly. Following injection of actin into Drosophila larvae, they observed induced sterile inflammation in the fat body. They identified that exogenous actin acts as a conserved signal that is released from damaged cells which leads to a selective JAK/STAT response [24]. While the actual actin receptor remains elusive (there is no fly homologue for the mammalian actin receptor DNGR-1 and Draper appears dispensable), the actin signal appears to feed into the SFK-Draper-Shark pathway further strengthening its evolutionary conservation as a cell injury detector which precedes the evolution of adaptive immunity.
NON-CANONICAL TOLL SIGNALING: A VERSATILE TOOL [25]
PERSEPHONE: BAIT FOR EXOGENOUS PROTEASES AND ENDOGENOUS SIGNALS
In trained immunity in mammals, innate immune cells can be primed by primary infections or vaccination to perform more efficiently upon subsequent exposure to microbial attack. Similarly, insect immunity can be primed by previous exposure to antigens, a phenomenon that has been dubbed “immune memory” or “immune priming” [31]. While trained immunity in mammals confers broad range protection against unspecific microbes, at least some cases of insect immune priming appear to be quite specific [32]. Though the possibility that the mechanism exists in vertebrates and organisms with an adaptive immunity has not yet been ruled out. While exogenous signals have been found responsible for immune training/priming, similarly in mammals and insects, tissue damage appears to play a central educational role for insect hemocytes [33]. Like macrophages, hemocytes are multifunctional cells taking care of both internal damage and microbial attack by for example, phagocytosing bacteria or removing apoptotic cells. During the removal of apoptotic cells, Weavers et al. [33] showed that Drosophila embryonic development is essential for priming hemocytes both to efficiently perform wound healing and to fight infections. Priming is triggered by calcium flashes which activate JNK signaling and subsequent induction of the apoptotic regulator Draper, a key molecule in wound healing. Consequently, inhibiting apoptosis as well as interfering with JNK signaling affects the inflammatory potentials of hemocytes, which is somewhat expected but surprisingly, their immune competence is hindered for example, they lose the ability to phagocytose Escherichia coli [33]. Trained immunity has also been demonstrated in the case of viral infections in the fruit fly. Cellular damage releases viral dsRNA which is subsequently phagocytised by plasmatocytes and eventually packaged into endosomes to transfer antiviral RNAi to other hemocytes [34]. Thus the mechanism for priming hemocytes for a specific viral infection initially bears the hallmarks of damage-induced clearance but leads to specific protection. This varies widely from what has thus far been shown in the adaptive immune system in the mammalian model, but demonstrates that the innate immune system can play a similar role in immune priming.
CANCER AND DANGER: A MULTILEVEL AFFAIR
DANGER IN THE NERVOUS SYSTEM: NEUROINFLAMMATION
A number of approaches have been used to induce damage in the fly’s nervous system (NS) which include the expression of human disease-causing gene polymorphisms in the NS [48] as well as through causing mechanical damage [49]. Upon severing axons that lead to either wings or legs, transcriptome profiling of the ventral nerve cord revealed two main pathways involved in other wounding scenarios, mainly Draper/AP-1 and Toll, were upregulated. In addition, Stat92E/draper/JNK/AP-1 activity was necessary for metalloproteinase-1 (MMP1) to successfully clear away debris of severed axons and for regeneration [49]. While this study demonstrated activation of stress was required for innate glial immunity, another study used a fly model of Ataxia-Telangiectasia to demonstrate that the NFkB factor, Relish and the induction of select immune genes were key culprits for neurodegeneration [48] in a non-canonical manner (the imd pathway was dispensable). When different models of neurodegenerative scenarios are compared, an immune signature is often identified. It has been proposed that this immune signature may actually reveal an equally important neuroprotective function of the “immune genes” since both DAMPs and damage clearance play key roles during infections and tissue healing, a pleiotropy that is often misrepresented during gene annotation [50,51]. An example of this duality which exists within genes’ function is provided by a member of a prototypical PRR family (PGRP-LC, a peptidoglycan receptor in the Drosophila imd pathway), which is also required for synaptic plasticity in mice [52]. Similarly, immune transcription factor isoforms have additional (including regenerative) functions in non-immune tissues [53,54].
CONCLUSION - DROSOPHILA OFFERS AN INTEGRATIVE VIEW OF IMMUNITY
ACKNOWLEDGEMENT
1. Krautz R, Arefin B, Theopold U (2014) Damage signals in the insect immune response. Front Plant Sci 5: 342.
2. Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA (1996) The dorsoventral regulatory gene cassette spaetzle/toll/cactus controls the potent antifungal response in Drosophila adults. Cell J Immunol 86: 973-983.
3. Gold KS, Bruckner K (2014) Drosophila as a model for the two myeloid blood cell systems in vertebrates. Exp Hematol 8: 717-727.
4. Wang Z, Wilhelmsson C, Hyrsl P, Loof TG, Dobes P, et al. (2010) Pathogen entrapment by transglutaminase-a conserved early innate immune mechanism. PLoS Pathog 2: e1000763.
5. Shaukat Z, Liu D, Gregory S (2015) Sterile inflammation in Drosophila. Mediators Inflamm 2015: 369286.
6. Stramer BM, Dionne MS (2014) Unraveling tissue repair immune responses in flies. Semin Immunol 4: 310-314.
7. Lesch C, Jo J, Wu Y, Fish GS, Galko MJ (2010) A targeted UAS-RNAi screen in Drosophila larvae identifies wound closure genes regulating distinct cellular processes. Genetics 3: 943-957.
8. Kenmoku H, Hori A, Kuraishi T, Kurata S (2017) A novel mode of induction of the humoral innate immune response in Drosophila larvae. Dis Model Mech 3: 271-281.
9. Abreu-Blanco MT, Verboon JM, Parkhurst SM (2014) Coordination of Rho family GTPase activities to orchestrate cytoskeleton responses during cell wound repair. Curr Biol 2: 144-155.
10. Verboon JM, Parkhurst SM (2015) Rho family GTPases bring a familiar ring to cell wound repair. Small GTPases 1: 1-7.
11. Weavers H, Liepe J, Sim A, Wood W, Martin P, et al. (2016) Systems analysis of the dynamic inflammatory response to tissue damage reveals spatiotemporal properties of the wound attractant gradient. Curr Biol 15: 1975-1989.
12. Wu SC, Liao CW, Pan RL, Juang JL (2012) Infection-induced intestinal oxidative stress triggers organ-to-organ immunological communication in Drosophila. Cell Host Microbe 4: 410-417.
13. Niethammer P, Grabher C, Look AT, Mitchison TJ (2009) A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 7249: 996-999.
14. Yoo SK, Starnes TW, Deng Q, Huttenlocher A (2011) Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 7375: 109-112.
15. Evans IR, Rodrigues FS, Armitage EL, Wood W (2015) Draper/CED-1 mediates an ancient damage response to control inflammatory blood cell migration in vivo. Curr Biol 12: 1606-1612.
16. Razzell W, Evans IR, Martin P, Wood W. (2013) Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr Biol 5: 424-429.
17. Kucerova L, Broz V, Arefin B, Maaroufi HO, Hurychova J, et al. (2015) The Drosophila chitinase-like protein IDGF3 is involved in protection against nematodes and in wound healing. J Innate Immun 2: 199-210.
18. Arefin B, Kucerova L, Dobes P, Markus R, Strnad H, et al. (2014) Genome-wide transcriptional analysis of Drosophila larvae infected by entomopathogenic nematodes shows involvement of complement, recognition and extracellular matrix proteins. J Innate Immun 2: 192-204.
19. Hauling T, Krautz R, Markus R, Volkenhoff A, Kucerova L, et al. (2014) A Drosophila immune response against Ras-induced overgrowth. Biol Open 4: 250-260.
20. Pendleton ED, Sullivan CJ, Sasmor HH, Bruse KD, Mayfield TB, et al. (2016) Actin exposure upon tissue injury is a targetable wound site-specific protein marker. Biochem Biophys Rep 7: 56-62.
21. Martinez Amat A, Marchal Corrales JA, Rodriguez Serrano F, Boulaiz H, Prados Salazar JC, et al. (2007) Role of alpha-actin in muscle damage of injured athletes in comparison with traditional markers. Br J Sports Med 7: 442-446.
22. Sandiford SL, Dong Y, Pike A, Blumberg BJ, Bahia AC, et al. (2015) Cytoplasmic actin is an extracellular insect immune factor which is secreted upon immune challenge and mediates phagocytosis and direct killing of bacteria and is a Plasmodium antagonist. PLoS Pathog 2: e1004631.
23. Ahrens S, Zelenay S, Sancho D, Hanc P, Kjaer S, et al. (2012) F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity 4: 635-645.
24. Srinivasan N, Gordon O, Ahrens S, Franz A, Deddouche S, et al. (2016) Actin is an evolutionarily-conserved damage-associated molecular pattern that signals tissue injury in Drosophila melanogaster. Elife 5. pii: e19662.
25. Lindsay SA, Wasserman SA (2014) Conventional and non-conventional Drosophila toll signaling. Dev Comp Immunol 1: 16-24.
26. Ming M, Obata F, Kuranaga E, Miura M (2014) Persephone/Spatzle pathogen sensors mediate the activation of toll receptor signaling in response to endogenous danger signals in apoptosis-deficient Drosophila. J Biol Chem 11: 7558-7568.
27. Kanoh H, Kuraishi T, Tong LL, Watanabe R, Nagata S, et al. (2015) Ex vivo genome-wide RNAi screening of the Drosophila toll signaling pathway elicited by a larva-derived tissue extract. Biochem Biophys Res Commun 2: 400-406.
28. Yamamoto-Hino M, Muraoka M, Kondo S, Ueda R, Okano H, et al. (2015) Dynamic regulation of innate immune responses in Drosophila by Senju-mediated glycosylation. Proc Natl Acad Sci U S A 18: 5809-5814.
29. Meyer SN, Amoyel M, Bergantinos C, de la Cova C, Schertel C, et al. (2014) An ancient defense system eliminates unfit cells from developing tissues during cell competition. Science 6214: 1258236.
30. Issa N, Guillaumot N, Lauret E, Matt N, Schaeffer-Reiss C, et al. (2018) The circulating protease Persephone is an immune sensor for microbial proteolytic activities upstream of the Drosophila toll pathway. Mol Cell 4: 539-550
31. Cooper D, Eleftherianos I (2017) Memory and specificity in the insect immune system: Current perspectives and future challenges. Front Immunol 8: 539.
32. Sadd BM, Schmid-Hempel P (2006) Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr Biol 12: 1206-1210.
33. Weavers H, Evans IR, Martin P, Wood W (2016) Corpse engulfment generates a molecular memory that primes the macrophage inflammatory response. Cell 7: 1658-1671.
34. Tassetto M, Kunitomi M, Andino R (2017) Circulating immune cells mediate a systemic RNAi-based adaptive antiviral response in Drosophila. Cell 2: 314-325.
35. Dvorak HF (1986) Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 26: 1650-1659.
36. Hernandez C, Huebener P, Schwabe RF (2016) Damage-associated molecular patterns in cancer: A double-edged sword. Oncogene 46: 5931-5941.
37. Pagliarini RA, Xu T (2003) A genetic screen in Drosophila for metastatic behavior. Science 5648: 1227-1231.
38. Brumby AM, Richardson HE (2003) scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J 21: 5769-5779.
39. Pastor-Pareja JC, Xu T (2013) Dissecting social cell biology and tumors using Drosophila genetics. Annu Rev Genet 47: 51-74.
40. Fogarty CE, Diwanji N, Lindblad JL, Tare M, Amcheslavsky A, et al. (2016) Extracellular reactive oxygen species drive apoptosis-induced proliferation via Drosophila macrophages. Curr Biol 5: 575-584.
41. Parisi F, Stefanatos RK, Strathdee K, Yu Y, Vidal M (2014) Transformed epithelia trigger non-tissue-autonomous tumor suppressor response by adipocytes via activation of Toll and Eiger/TNF signaling. Cell Rep 5: 855-867.
42. Perez E, Lindblad JL, Bergmann A (2017) Tumor-promoting function of apoptotic caspases by an amplification loop involving ROS, macrophages and JNK in Drosophila. Elife 6. pii: e26747.
43. Halme A, Cheng M, Hariharan IK (2010) Retinoids regulate a developmental checkpoint for tissue regeneration in Drosophila. Curr Biol 5: 458-463.
44. Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, et al. (2004) The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 7: 730-737.
45. Ostwald TJ, MacLennan DH (1974) Isolation of a high affinity calcium-binding protein from sarcoplasmic reticulum. J Biol Chem 3: 974-979.
46. Somogyi E, Petersson U, Hultenby K, Wendel M (2003) Calreticulin - An endoplasmic reticulum protein with calcium-binding activity is also found in the extracellular matrix. Matrix Biol 2: 179-191.
47. Kuraishi T, Manaka J, Kono M, Ishii H, Yamamoto N, et al. (2007) Identification of calreticulin as a marker for phagocytosis of apoptotic cells in Drosophila. Exp Cell Res 3: 500-510.
48. Petersen AJ, Katzenberger RJ, Wassarman DA (2013) The innate immune response transcription factor relish is necessary for neurodegeneration in a Drosophila model of ataxia-telangiectasia. Genetics 1: 133-142.
49. Purice MD, Ray A, Munzel EJ, Pope BJ, Park DJ, et al. (2017) A novel Drosophila injury model reveals severed axons are cleared through a Draper/MMP-1 signaling cascade. Elife 6. pii: e23611.
50. Venereau E, Ceriotti C, Bianchi ME (2015) DAMPs from cell death to new life. Front Immunol 6: 422.
51. Cantera R, Barrio R (2015) Do the genes of the innate immune response contribute to neuroprotection in Drosophila? J Innate Immun 1: 3-10.
52. Harris N, Braiser DJ, Dickman DK, Fetter RD, Tong A, et al. (2015) The innate immune receptor PGRP-LC controls presynaptic homeostatic plasticity. Neuron 6: 1157-1164.
53. Zhou B, Lindsay SA, Wasserman SA (2015) Alternative NF-kappaB isoforms in the Drosophila neuromuscular junction and brain. PLoS One 7: e0132793.
54. Tang X, Zhao Y, Buchon N, Engstrom Y (2018) The POU/Oct transcription factor nubbin controls the balance of intestinal stem cell maintenance and differentiation by isoform-specific regulation. Stem Cell Rep 5: 1565-1578.
55. Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J (2017) Neuroimmunology of traumatic brain injury: Time for a paradigm shift. Neuron 6: 1246-1265.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Journal of Spine Diseases
- Journal of Alcoholism Clinical Research
- International Journal of AIDS (ISSN: 2644-3023)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)